During World Antimicrobial Awareness Week, advocacy focuses on the many bacteria species that cause disease that have grown resistant to modern antibiotics.
At the University of Guelph, Dr. Rebecca Shapiro, associate professor in the Department of Molecular and Cellular Biology in the College of Biological Science, takes a different approach. In Shapiro’s lab, graduate students and other researchers study microbial fungal pathogens.
In late 2022, the World Health Organization released the first-ever list of fungal priority pathogens, a record of 19 fungi considered the greatest threat to public health.
Bacterial pathogens have been well-studied by scientists, while fungal pathogens have not received the same kind of attention despite their ever-growing impact on human health.
What is antimicrobial resistance?
Antimicrobial resistance is the ability of microbial species to evolve resistance and survive in the presence of drugs used to get rid of them.
“Overwhelmingly, when we talk about antimicrobial resistance, we talk about antibiotic resistance, which is more specifically resistance of bacteria to antibiotic drugs,” Shapiro says. “In my lab, we work on another corner of this that is less discussed, which is the resistance of fungal pathogens to antifungal drugs.”
What are fungal pathogens?

The most common fungal pathogen affecting humans is Candida, a family of yeast species that cause severe illness.
Amongst these Candida species, Candida auris, first documented in 2009, is an emerging pathogen now found all over the world. It spreads easily and some strains are resistant to all three major classes of antifungals. Large outbreaks were seen during the COVID-19 pandemic, largely because of the way it spreads in health care settings.
Fungi are natively associated with human bodies, meaning they live naturally on the skin and in the mouth, but in immunocompromised people, Candida can overgrow and cause life-threatening mucosal and blood infections.
Some fungal species are becoming increasingly important pathogens that cause what Shapiro calls “opportunistic infections,” or diseases that target humans with weakened immune systems or other types of underlying vulnerabilities.
“We started to appreciate this during COVID,” she says, of higher-risk populations.
This becomes problematic, she explains, because of the increase in the number of people who live with underlying disease. Ironically, medical breakthroughs in other realms – organ transplants, cancer therapies – leave people immunocompromised, and vulnerable to fungal disease.
There is also evidence to suggest global warming is affecting the emergence and spread of fungal pathogens, Shapiro says. “This is the broader context in which we’re studying fungi.”
Difficulties in developing antifungal therapies
Bacteria are primitive, distant organisms, whereas fungi are closely related to animals, Shapiro says. As a result, their fundamental biology – their cells – are surprisingly similar to humans.
“When trying to develop antifungal therapies, it can be incredibly difficult because the whole point of a drug is to kill the fungal cell without being toxic to the human,” Shapiro says. “It’s really hard to find those drugs because of how similar the two are.”
As a result, there are very limited anti-fungal drugs. If resistance starts to occur to one, options quickly decrease.
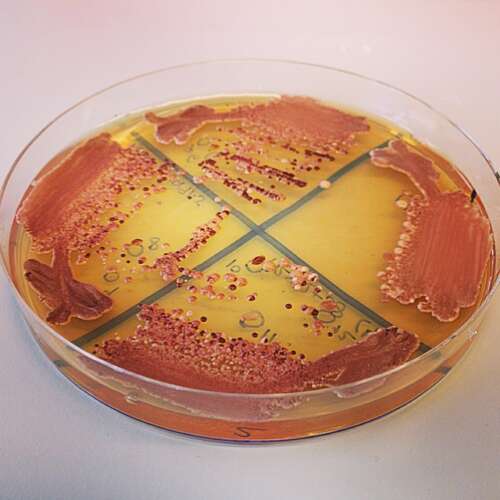
“In my lab, we’re interested in various aspects of this,” she says.
Some projects focus on developing new antifungal drugs, while others are researching what occurs in a fungal cell at a genetic level to make it resistant.
The students investigate new targets within fungal cells that could make them less resistant. They are not necessarily looking for breakthrough cures but a more comprehensive understanding of what is occurring under the microscope.
“We grow cells in large batches and subject them to different doses of antifungals. You can actually see with your naked eye where they grow and where they can’t grow,” she says, thereby revealing how sensitive or resistant they are.
The team uses the gene editing tool CRISPR, to analyze the function of each fungal gene and uncover what role they play in helping fungi multiply as well as the pathways they use to resist treatment with antimicrobials.
Working in the realm of genetics, the work becomes more intangible. DNA sequencing enables the team to assess cells they are unable to see, introducing mutations to the genome to see how that alters resistance.
“It’s microbiology on one hand,” she says, “but also molecular biology.”
The pressing concern, of course, is what happens when all therapies are exhausted. There are three kinds of antifungals, some used sparingly for resistant infections.
“This is why the focus is on both understanding drug resistance itself, but also pushing forward the development of new drugs.”
Contact:
Dr. Rebecca Shapiro
shapiror@uoguelph.ca